We have recently developed a new voltage sensor, Ace-mNeon, that is able to detect action potentials in live animals [local abstract][Full article at Science Magazine]. The field of GEVIs has been ongoing for almost two decades now, with the goal of being able to measure voltage signals in live animal models. There has been many developments within the past few years, each inching closer toward the goal of being able to detect action potentials in live model organisms. Much like our previous FRET-opsin sensors, we fused a bright fluorescent protein to a fast voltage sensing domain. This resulted in a sensor with 5-6 times faster kinetics than our previous MacQ-mCitrine sensors, and approximately twice as bright.
With the development of this sensors, we've demonstrated for the first time optical detection of spiking activity with a GEVI in an awake, behaving mice and flies (Fig. 1). In addition, we demonstrated that we can use this sensor to examine the propagation of voltage in sub-cellular compartments as well (Fig. 2). Although we can only do this in a small number of neurons simultaneously, breaking across the threshold of doing live animal experiments suggests that there are useful experiments that neuroscientists can immediately attempt in the near future. Our development also suggests that large-scale parallel voltage imaging will be possible farther in the future.
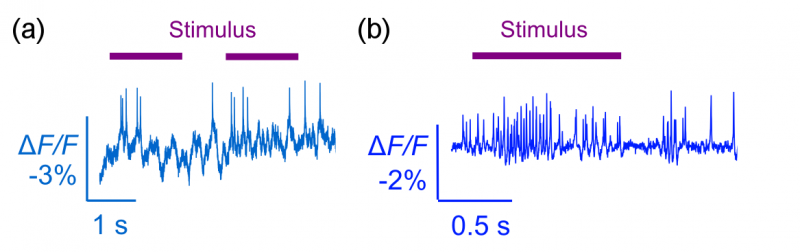
Fig 1 - (a) Optically detected spikes from an awake behaving mouse driven by visual stimulus. (b) Optically detected spikes from an awake behaving fly drive by odor stimulus.
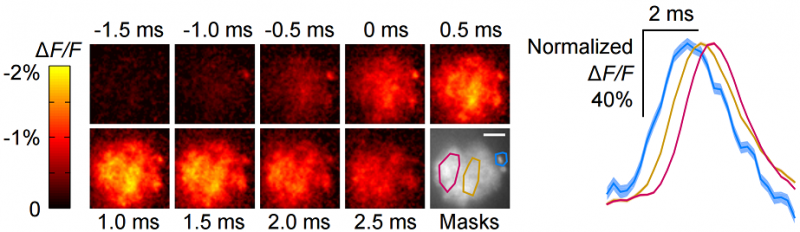
Fig 2 - Voltage propagation throughout a fly odor neuron. The voltage initially starts in the cell body (upper right of each panel), and propagates leftward through the periphery of the neuron. Masked regions at the soma (cyan) and neuron process (gold, magenta), show temporally offset voltage activity (right inset).


